An Update On The Medical Field
May 26, 2020
In light of the coronavirus pandemic, scientists and doctors are working against the clock to discover a possible cure while following several leads.
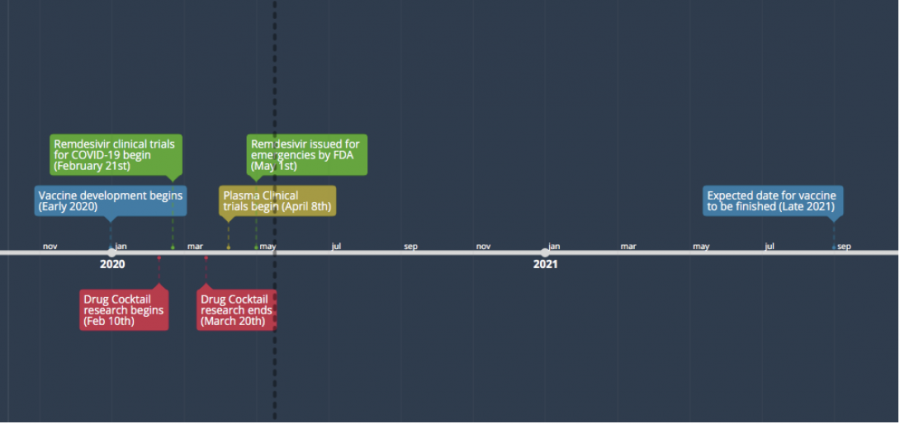
In terms of funding, the research against coronavirus is already well-situated. The funds are all funneled towards one goal: vaccines. American companies have already pledged $7 billion, along with $1.1 billion from European nations and Norway. Bill Gates, partnered with Wellcome and Mastercard, has already funneled $125 million in an effort to identify and assess the virus quickly. Sadly, these donations won’t make a vaccine pop out of thin air. The likelihood of a vaccine being developed in 2020 is relatively low. Researchers expect one as late as 2021, or even 2022.
Fortunately, we already have a head start, that being the research we have of SARS and MERS. SARS and COVID-19 are 80% identical, allowing scientists to develop tests quicker. However, moving at such a brisk pace may also cause problems. The chances of failure will increase if we blow through the research phase.
Despite this, cell biologist Robert Van Exan has stated that moving quickly is “worth the try — maybe we will get lucky.”
Continuing at a brisk pace, medical organizations have already begun to test vaccines. Pfizer, a pharmaceutical corporation based in New York, has started injecting a select few human volunteers with four different variations of the vaccine and wishes to expand testing to hundreds of more people.
The CEO and Chairman Albert Bourla stated, “If one or two variations indicate success, the company will ramp up trials, and then in September launch a broad-scale study with thousands of participants if a vaccine proves to be successful.”
While scientists are attempting to discover a vaccine to prevent the virus, they’ve also shifted their attention to those who have already contracted it.
Those who’ve recovered from the coronavirus are able to assist in the fight by making a valuable donation to scientists: their blood.
The plasma donated from those who have already overcome COVID-19 is being studied by scientists as well. Containing antibodies that help fight off infection should it arrive again, it’s invaluable in both developing a vaccine and protecting those who already have the virus.
The transfusion of plasma has been proven to decrease the recovery time of sick patients, but it’s still being researched to be sure that it is safe and effective. Tom Hanks, who was diagnosed with the virus a few months ago, has also decided to assist scientists by donating his plasma.
Hanks tweeted, “Here’s last week’s bag of plasma. Such a bag! After the paperwork, it’s as easy as taking a nap,” accompanied by a photo of a package of plasma.
Another drug used for the COVID-19 pandemic is Remdesivir, which received emergency approval from the FDA. An antiviral drug made by the company Gilead, Remdesivir kills a wide variety of viruses by inhibiting their growth and development. However, people are still suspicious about the drug. This stems from one of its side effects: the increase of liver enzymes, which can lead to damaged liver cells and infusion-related reactions such as low blood pressure.
Remdesivir is distributed only in emergencies to treat patients classified with severe disease, defined as patients with low blood oxygen levels, or who require breathing support through a ventilator. Nevertheless, it has been shown to have a positive effect compared to a placebo, and is starting to be used in hospitals at the time of writing.
Dr. Fauci, director of the National Institute of Allergy and Infectious Diseases, said, “The data shows that Remdesivir has a clear-cut, significant, positive effect in diminishing the time to recover.”
Finally, there is a certain mixture of drugs that seems to speed up recovery time for COVID-19 patients. In a clinical trial conducted in Hong Kong, it was shown that three antiviral drugs could be used in combination to ease symptoms. The drugs consist of an HIV treatment drug lopinavir-ritonavir, a hepatitis treatment drug ribavirin, and a multiple sclerosis drug interferon.
In its first stages of testing, a control group and a placebo group were tested, but the control group recovered five days earlier than the placebo group. While this treatment isn’t groundbreaking, it will allow hospitals to let patients recover a lot more quickly.
The drugs were “superior to lopinavir–ritonavir alone in alleviating symptoms and shortening the duration of viral shedding and hospital stay in patients with mild to moderate COVID-19,” according to Kwok-Yung Yuen, a doctor who led the research.
The race against the coronavirus is picking up pace, funded by an entire world desperate to stop it. Researchers and scientists are working their hardest to discover a cure, and it’s up to us to stay inside in order to give scientists peace of mind while they save our lives.